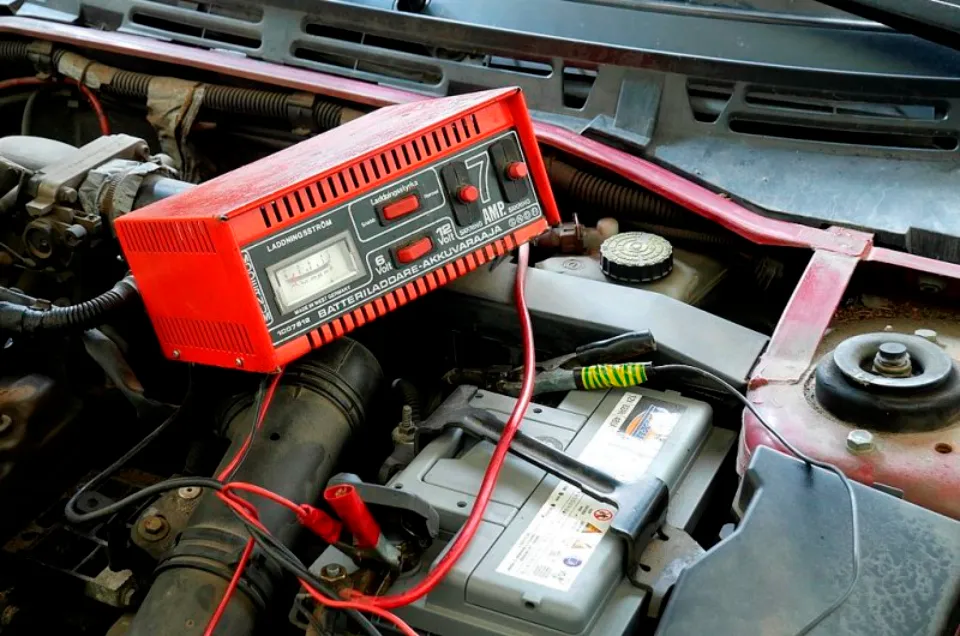
Lead-acid battery also referred to as a secondary battery, rechargeable battery, rechargeable battery, and primary battery. Since its invention in 1958, the lead-acid battery has been used and developed. It is a common component of the power end of diesel locomotives and has a history dating back more than 100 years.
The main development directions for the lead-acid battery used in new energy vehicles are to increase specific energy and cycle life. A relatively established new energy battery technology is the lead-acid battery.
Basic Introduction
Lead Acid Batteries (“LAB”) are considered the most cost-effective, safe, and environmentally friendly form of power storage. Lead, acid, and plastic—all of which are recyclable and separable—make up LAB’s three main components.

Large-format lead-acid designs are frequently used for storage in stand-alone power systems, high-availability emergency power systems like hospitals, and backup power supplies in cell phone towers. It may be possible to use modified versions of the standard cell for these roles to increase storage capacity and lower maintenance needs. Gel cells and absorbed glass-mat batteries are common in these roles, collectively known as Valve-regulated lead-acid (VRLA) batteries.
Advantages of Lead-acid Batteries
In addition to lithium-ion batteries, among the on-site batteries, the voltage of lead-acid batteries is relatively high, which is 2.0V; check out lead acid battery vs. lithium-ion.
Low manufacturing cost;
It can be made into batteries of various sizes and structures ranging from 1Ah to several thousand Ah;
The high-rate discharge performance is good, and it can be used for engine starting;
The power efficiency can reach 60%;
Good high and low-temperature performance, can work under the condition of -40℃~60℃;
It is easy to use for floating charging, has no “memory” effect, and is easy to identify the state of charge.
Disadvantages of Lead-acid Batteries
The specific energy is low, the overall mass and volume required by the weight of the new energy vehicle are relatively large, and the journey that can be driven on a single charge is relatively short;
The lifespan of lead acid batteries is short and the cost of later use is high;
Long charging time;
Lead is a heavy metal and pollutes, which runs counter to the concept of new energy vehicles.

How Does a Lead-Acid Battery Work?
The working principle of lead acid battery is easy. Simply put, when the sulfate in the sulphuric acid bonds to the lead, the battery’s electrical charge is produced. Reversing this reaction will replenish the electrical charge. In other words, the sulfate reacts with sulphuric acid once more to recharge the battery. The sulfate ions in the acid are obviously limited in number.

Additionally, the lead it bonds to has a finite surface area. Consequently, the charge deteriorates as the sulfate is consumed.
Because of this, lead-acid batteries are not recommended for continuously operating devices. They work best, however, for applications that demand a quick burst of strength.

Plate Vulcanization
The so-called vulcanization refers to the formation of irreversible lead sulfate salinization on the positive and negative plates to form a layer of white coarse-grained crystalline lead sulfate. It is challenging to get rid of this crystal during routine charging.

There are many reasons for the vulcanization of the plate, but the main ones are that lead-acid batteries are stored for too long periods of time, that sulfuric acid is present on the surface of the active material when the plate is being formed, and that lead sulfate on the surface of the active material loses its ionization effect over time.

Lead Acid Battery Vs Lithium-ion
The top three distinctions between lead acid battery and lithium-ion are listed below, along with some examples of which technology to choose when going electric.
Cost
Lead acid is a popular cost-effective battery chemistry, available in large quantities with little worries relating to the security of the supply and in a variety of off-the-shelf pack sizes. When space is at a premium and energy needs are minimal, lead acid is a fantastic fit for large-scale stationary applications.

Energy and Range
When comparing the two chemistries side by side, lithium-ion achieves an energy density of 125-600+ Wh/L versus 50-90 Wh/L for lead acid batteries. The lead acid battery, which is heavier and could require up to 10 times the volume of the lithium-ion battery to travel the same distance, would also require more space if you used both types of lead acid batteries in the same type of vehicle.
Charging
A lithium-ion battery can charge in 3 hours or as little as a few minutes, depending on the battery’s size. But it takes 10 hours to fully charge a lead-acid battery. Lead-acid batteries need to be charged more slowly than lithium-ion batteries because the former can handle higher current rates.

Conclusion
After getting the answers to your queries, use this guide to decide whether lead-acid batteries are the best option for your requirements. Also, do you know anyone else who’s wondering, “The way a lead-acid battery functions?” If so, please let them know about this manual. If you want to learn more about lead acid batteries, please check Power Your Feed website.